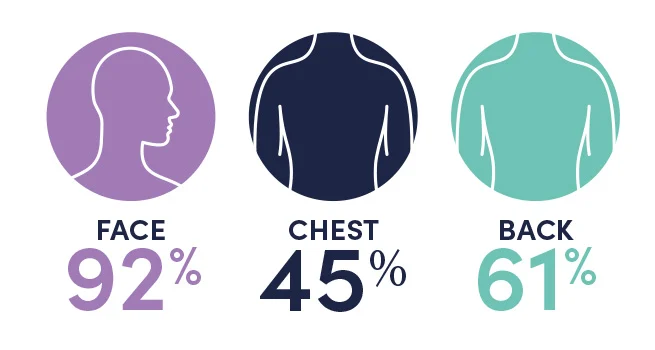
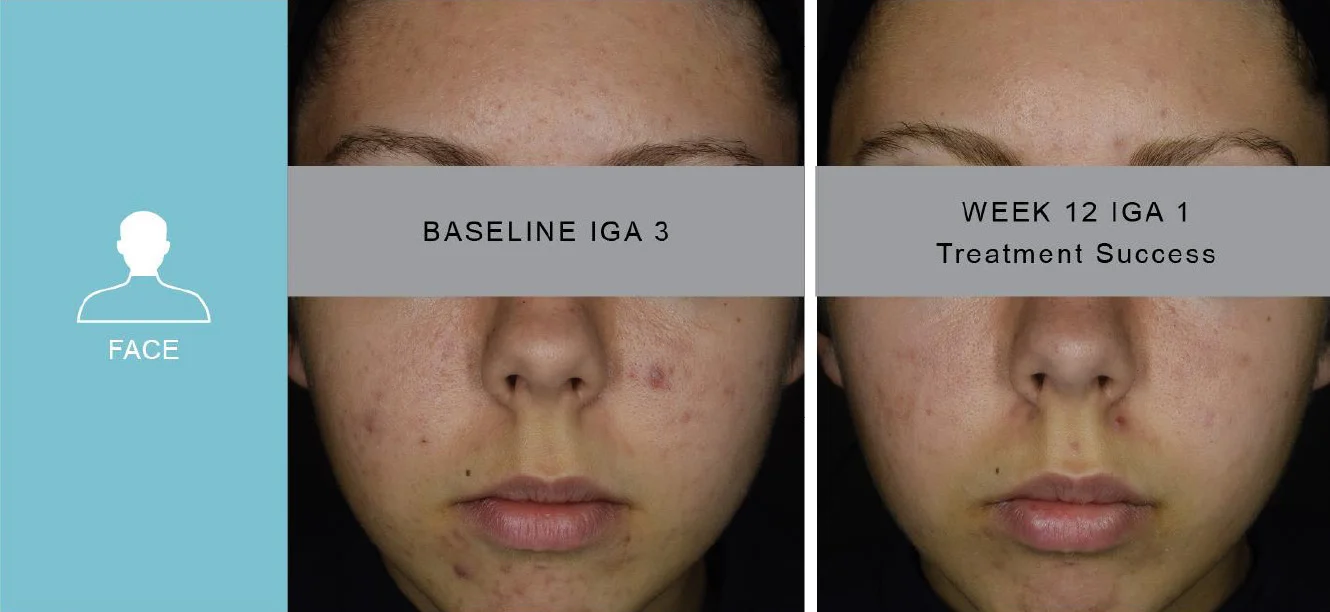
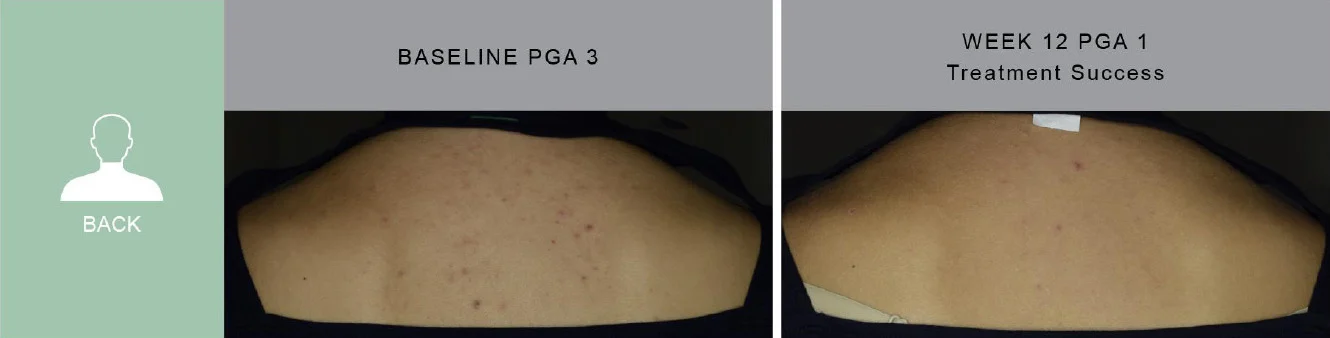
*PIVOTAL Study 1 results vs baseline at Week 12
** Representative of study population with photos during study
† P less than 0.001 versus vehicle cream
‡ % of subjects with an IGA score of clear (0) or almost clear (1) and at least a 2-grade improvement from baseline to Week 12
IGA: Investigator Global Assessment; IL: Inflammatory lesion; NIL: non-inflammatory lesion; PGA: Physician Global Assessment; RAR-α: retinoic acid receptor alpha; RAR-β: retinoic acid receptor beta; RAR-γ: retinoic acid receptor gamma.
References: 1. Kasser M et al. J Cosmet Dermatol. 2020;00:1–6. 2. Stein Gold L et al. Why We Should Consider Evidence-Based Treatment Options for Truncal Acne. Dermatol Ther (Heidelb) 2021;11:661–664. 3. Blume-Peytavi U et al. Long-term safety and efficacy of trifarotene 50 μg/g cream, a first-in-class RAR-γ selective topical retinoid, in patients with moderate facial and truncal acne. JEADV 2020, 34, 166–173. 4. Bell K et al. Tretinoin 0.1% and Benzoyl Peroxide 3% Cream for the Treatment of Facial Acne Vulgaris. Annals of Pharmacotherapy 2021;55(1) 111–116. 5. Aklief Cream Summary of Product Charactistics. Available at: https://www.medicines.org.uk/emc/ product/13881/smpc#gref. Accessed: February 2025. 6. Tan J et al. Randomized phase 3 evaluation of trifarotene 50g/g cream treatment of moderate facial and truncal acne. J Am Acad Dermatol. 2019 Jun;80(6):1691-1699. 7. Aubert J et al. Nonclinical and human pharmacology of the potent and selective topical retinoic acid receptoragonist trifarotene. Br J Dermatol. 2018 Aug;179(2):442-456. 8. Fisher GJ et al. Immunological Identification and Functional Quantitation of Retinoic Acid and Retinoid X Receptor Proteins in Human Skin. JBC 1994;269;32:20629-20635. 9. Cosio T et al. Trifarotene: A Current Review and Perspectives in Dermatology. Biomedicines 2021, 9(3), 237; https://doi.org/10.3390/biomedicines9030237. 10. Zasada M, Budzisz E. Retinoids: active molecules influencing skin structure formation in cosmetic and dermatological treatments. Postepy Dermatol Alergol. 2019 Aug 30;36(4):392–397. doi: 10.5114/ada.2019.87443. 11. Tan J, Beissert S, Cook-Bolden F, Chavda R, Harper J, Hebert A, Lain E, Layton A, Rocha M, Weiss J, Dréno B. Impact of facial and truncal acne on quality of life: A multi-country population-based survey. JAAD Int. 2021;3:102- 110. 12. Del Rosso JQ, Bikowski JB, Baum E, Smith J, Hawkes S, Benes V, Bhatia N. A closer look at truncal acne vulgaris: prevalence, severity, and clinical significance. J Drugs Dermatol. 2007;6(6):597-600. 13. Tan J et al. Prevalence and severity of facial and truncal acne in a referral cohort. J Drugs Dermatol. 2008;7(6):551-6. 14. UK Data on File 015 - Subject photographs from Study 18251